2012’s ozone hole season starts
As an annually recurring phenomenon the Antarctic ozone hole is a dramatic illustration of how people change Earth’s atmosphere, thereby threatening life on the surface.
The so-called ozone layer is to be found at about 20 to 30 km altitude. It forms a natural protective screen against the dangerous ultraviolet radiation in sunlight. Its thickness can be reduced dramatically by the weather in the upper atmosphere, and also by chemical processes. Since the 1970s it has been known that substances released by human activity can damage the stratospheric ozone layer. Especially the chlorine compounds commonly found in coolants and fire extinguishers have turned out to be destroyers of ozone. To keep the ozone hole from expanding (small holes are also appearing in springtime increasingly over northern Europe), those countries which signed the Montreal Protocol in 1987agreed to prohibit the use of substances which damage the ozone layer. Since the atmosphere is an extremely slowly reacting system, it took until the late 1990s for the ozone hole to reach its maximal extent, despite the ban.
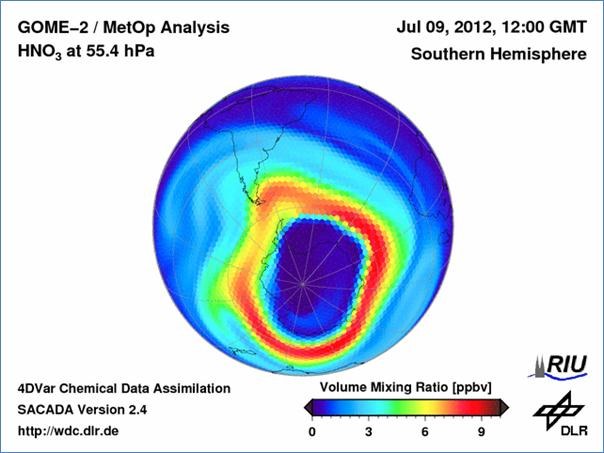
These July days, chemical processes have already started to prepare the way for the dramatic disintegration of the ozone layer over the Antarctic which will become visible in September and October. The figure shows the current distribution of nitric acid at 20 km altitude on 9 July 2012, as estimated by scientists at DLR’s German Remote Sensing Data Center based on satellite data. A large dark blue area with a very low gas concentration can be clearly noted over the Antarctic. It marks the position of the south polar vortex, an enormous cyclone over the South Pole. Here the temperatures are so low in winter that nitric acid droplets and ice particles can form, thereby removing nitrogen radicals from the chemical reactor of the stratosphere. This weakens the natural “immune system” of the atmosphere because nitrogen radicals can neutralize the chlorine released by human activity into the atmosphere and in this way hinder the destruction of ozone, which starts as the sun rises. In the deep blue area the ozone is already helplessly at the mercy of the springtime sunshine. Presumably, there will be a large ozone hole also this year.
For an ozone hole, the layer is reduced in thickness by more than 50%. Halving the thickness of the ozone hole leads to a doubling of the UV radiation reaching Earth’s surface. If our skin is exposed to UV-B (280-315 nm) radiation for a significant length of time, genetic defects in the skin cells can be the result. Plants die, sheep go blind, etc.
Together with the Association of German Dermatologists, DLR operates a UV alert service which gives practical advice about how long skin of a given type can tolerate exposure to the sun: see link.
