AVLase
In the AVLase cooperation project with the Institute of Aerospace Medicine and industrial partners, a laser-based rapid test for viral infections is being developed and demonstrated using the example of SARS-CoV2. The measurement principle and market potential were tested as part of a feasibility study with positive results.
Brief Description
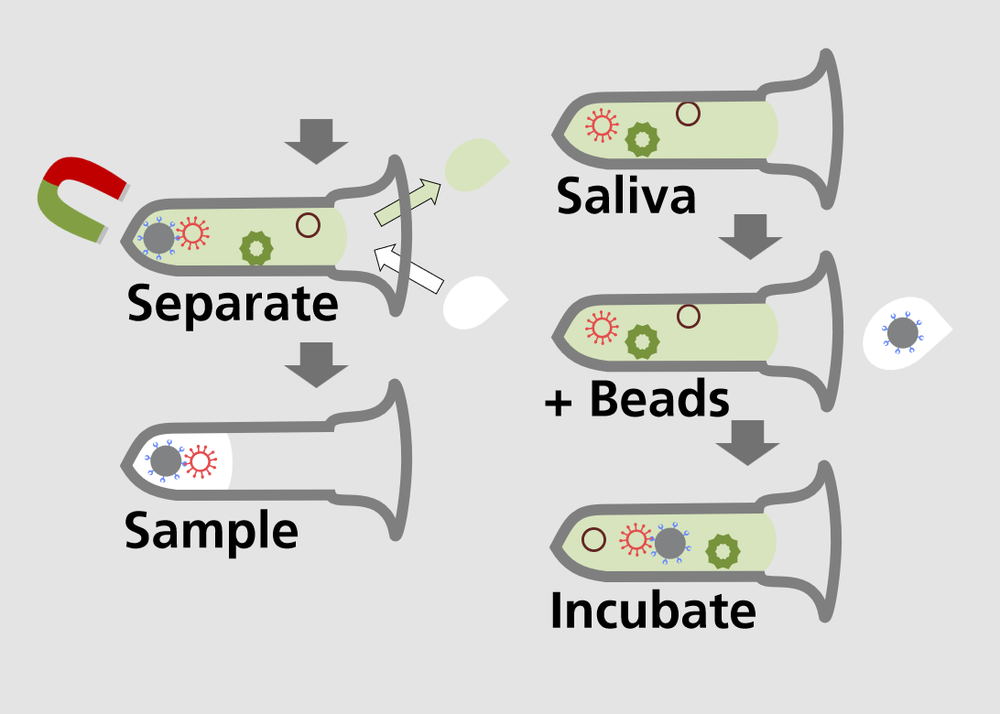
AVLase is a rapid test for the detection of viral infections in saliva samples. The samples are freed from impurities using a cleaning process specially tailored to the virus in question. An infrared laser scans the sample and takes a spectral fingerprint, which is used to identify the virus. This two-stage process results in a low detection limit with a low false alarm rate.
Applications
Application in test centres, airports and at events
Testing without medical professionals
Automated sample management with parallelised sample preparation and disposal for maximum throughput
Extension of the test spectrum to other virus types, e.g. influenza Extension of the sample spectrum from saliva to e.g. blood or urine samples
Facts and figures
Saliva test: simple sampling
Result after 5-10 minutes
300-400 tests per hour
Low detection limit: < 1 x 105 viruses / mL
High hit rate with low false alarm rate
Direct spectroscopic detection
Rapid adaptation to new viruses
The technology enables the rapid, centralised processing of large test quantities and the simple sample collection and automated preparation means that the test can be carried out contact-free by trained personnel. the core technology is the direct spectroscopic detection of the virus. To extend the test spectrum to other viruses, it is therefore only necessary to adapt the purification procedure: Magnetic nanoparticles are functionalised with specific binding partners tailored to the target virus. The virus binds to the particles according to the lock-and-key principle, thus enabling purification and enrichment of the sample.
The AVLase project is supported by DLR Technology Marketing (VO-TF) in order to ensure a rapid transition to the market.
