REDHy
In the REDHy research project, researchers at the DLR Institute of Technical Thermodynamics and their partners are developing a newly designed electrolyser. The REDHy approach is highly adaptable, durable, environmentally friendly, inherently safe, cost-efficient and enables the production of green hydrogen at high current densities. In contrast to SoA electrolysers, REDHy is completely free of critical raw materials and does not require fluorinated membranes or ionomers. The aim is to develop a prototype that can be operated in a laboratory environment under the agreed conditions (TLR4).
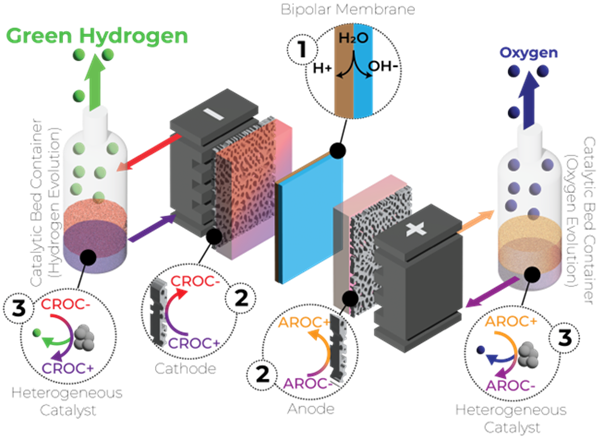

Redox mediator mediated, cost-effective and highly efficient technology for the production of green hydrogen without critical materials
The REDHy system is based on the principle of the redox flow battery. Redox mediators are used as the electrolyte. The innovative approach is to separate hydrogen and oxygen production in a three-stage process. The three stages are divided into 1) water dissociation, 2) electrical storage and 3) green hydrogen and oxygen production via a heterogeneous chemical reaction. Through the water dissociation step and the redox mediator reactions, REDHy bypasses the electrochemically inert water splitting process, resulting in lower overvoltage and low-cost materials. Costs can be reduced by avoiding the use of critical raw materials such as titanium and platinum. The system should also allow the use of seawater.
Decoupled hydrogen and oxygen production
Decoupling gas generation from the cell by transferring it to an external container helps to increase safety. For example, hydrogen and oxygen production can take place outside a building. The gas is produced in the container by the reaction on a heterogeneous catalyst. Water is first split into a proton and a hydroxide ion at the bipolar membrane. The protons react on the hydrogen side of the heterogeneous catalyst to form hydrogen. On the oxygen side, the hydroxide ions react on the heterogeneous catalyst to form oxygen and water.
Technical objectives of the project
- Development of a large-area short stack with 5 cells
- Active area of 100 cm²/cell
- Nominal power of 1.5 kW
- Energy consumption of 48 kWh/kg hydrogen
- Energy efficiency of over 82 %
- Nominal current density of 1.5 A/cm²
- Degradation rate of 0.1 %/1000 hours
Project | REDHy |
|---|---|
Duration |
|
Project participants |
|
Support | EU-funded: Horizon Europe |
Further links: |
