Low Temperature Electrolysers
Electrolysers are electrochemical devices that generate hydrogen and oxygen from splitting water. If they use renewable electricity, the produced hydrogen is green hydrogen. Hydrogen is used in fuel cells for producing electricity, but it is also used for many large industrial applications such as the chemical, steel and fertiliser industry. Large scale electrolysers have a service life of about 80.000 hours but different factors such as water impurities or rapid shut down can reduce it. Additionally, hydrogen crossover into the oxygen stream can pose a safety risk.
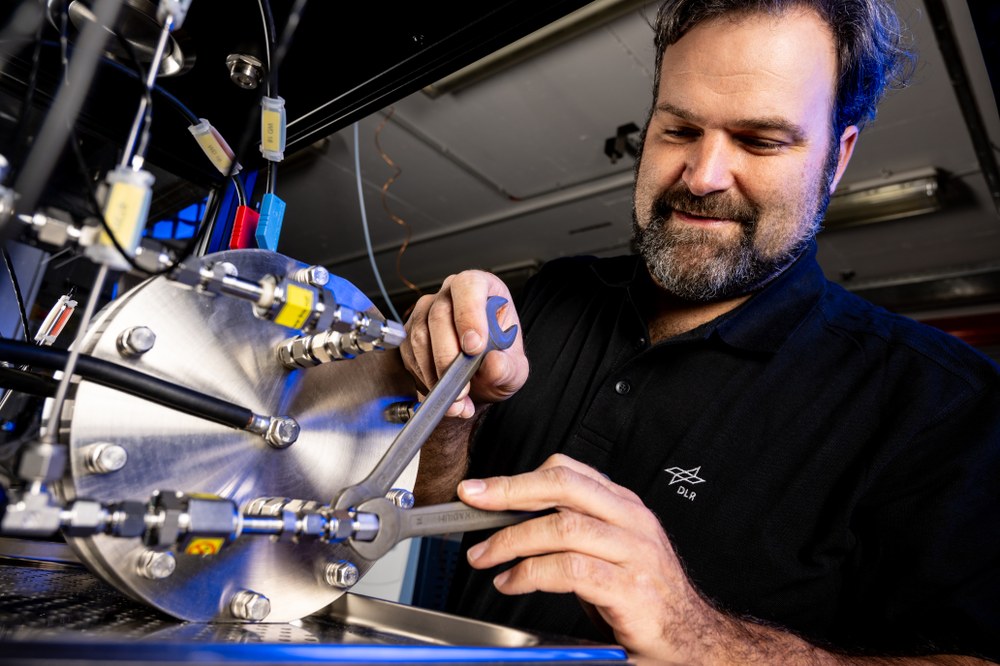
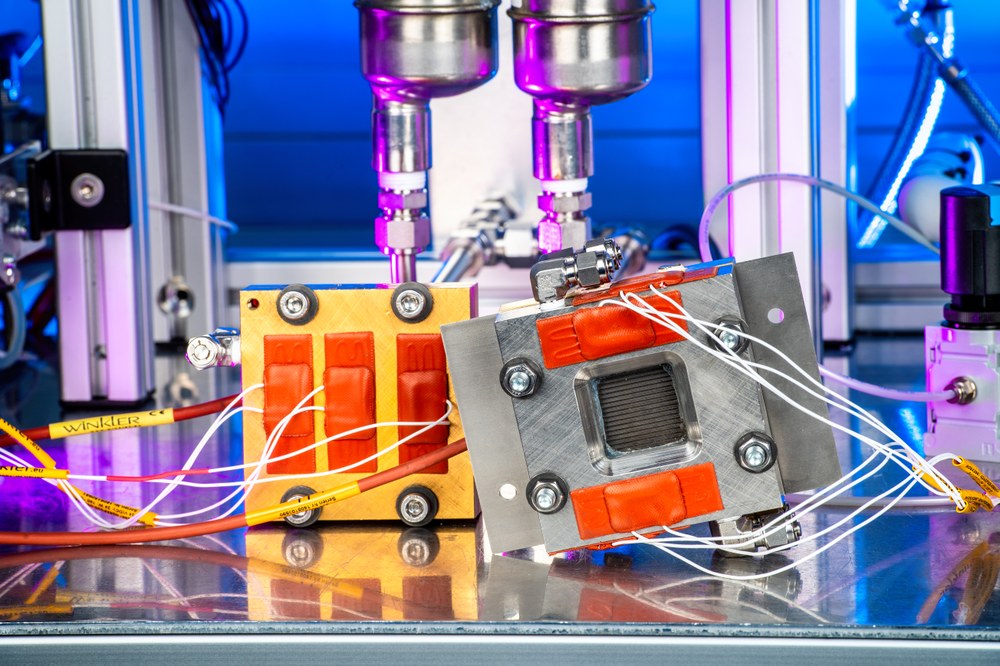
Fields of application
There are mainly three kind of low temperature water electrolysers. Alkaline electrolysis (AEL) is a long-time established technology that uses a liquid alkaline electrolyte, but it is limited in the range of operation due to the low current density.
Proton exchange membrane water electrolysers (PEMEL) on the other hand only need high purity water and can operate at high current densities. However, because of the acidic environment this technology requires precious metal as catalysts for the electrodes, which increases cost and limits upscaling.
Anion exchange membrane water electrolysis (AEMEL) is an emerging technology combine some of the advantages of the two previous ones. The use of a membrane in this technology allows to operate at high current densities but without requiring precious metals in the electrodes as the electrochemical environment is alkaline.
From the two first two electrolyser technologies, there are already system installed in the MW size in large industries. The third electrolyser technology is still very much in R&D.
Expertise
To lower the cost of green, that is hydrogen produced by electrolysers that use renewable electricity, the cost of the electrolyser must be decreased but without compromising performance and lifetime. Alkaline electrolysers require more active, efficient and stable electrodes that can allow increasing current densities. In the PEMWE, is necessary to reduce the amount of the precious metal used in the electrodes and protective coatings to lower the stack cost. Rapid, scalable and on-step production technologies such as flame spray pyrolysis allow producing anode catalysts with reduced amount of iridium.
Another spraying technology at DLR: Thermal spraying is being used to produce a titanium macro-porous layer (MPL) and use it at the interface between the porous transport layer (PTL) and the anode catalyst layer. The MPL not only protects the stainless steel PTL against corrosion, but it improves the performance and the lifetime of the PEMWE. Such MPL made of nickel instead of titanium is also being used in AEMWE having similar benefits as in PEMWE.
Degradation mechanisms are investigated using non-conventional physical techniques such as atomic force microscopy (AFM) and near ambient pressure X-ray photoelectron microscopy (NAP-XPS). With AFM it is possible to determine the loss of ionomer in the electrodes for PEMWE and AEMWE. NAP-XPS allows studying the changes of oxidation states of the catalysts in the electrochemical cell while they are in operation in a pressure reduced environment. The use of NAP-XPS can help to determine electrochemical reaction mechanisms.
